Advancing The Next Paradigm Shift in the Treatment of Onychomycosis
FDA approval of systemic antifungals in the nineties represented the first paradigm shift in the treatment of onychomycosis. However, the topical products that followed failed to match the effectiveness of orals because of their inability to reach nail bed infection sites in adequate therapeutic concentrations to eradicate fungal pathogens. Hallux Inc. is pioneering a new pharmacologic approach with Hallux Subungual Gel (HSG), a terbinafine dosage form applied topically onto the nail bed by physician. This route of administration utilizes subungual space, a tiny air gap accessed at the free edge of the toenail that provides an atraumatic pathway to mycotic tissue. HSG is undergoing a rigorous phase 2a efficacy, safety, and pharmacokinetics study evaluating once-monthly and once bi-monthly HSG applications over 44 weeks. The study nears completion and data permitting, will lead to the conduct of well-controlled studies, the final stage of clinical investigation required to apply for approval by the Food and Drug Administration.
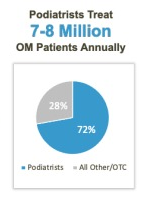
The risk of becoming infected with onychomycosis increases with age. Over eight million patients seek medical care each year for this unsightly and often painful nail disorder, the majority of whom visit a podiatrist or dermatologist. The most effective antifungal remains oral terbinafine (Lamisil®), dosed daily for three months. Topical antifungals are preferred due to the absence of systemic side effects and in particular liver damage. However, the issue for patients is that only a handful are FDA approved and none work very well.
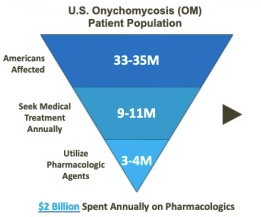
Moreover, the newer brands are expensive, poorly reimbursed, and must be self-administered (usually bedtime) every day for twelve months or more. Most patients give up. Hallux hopes to fill this gap with clinically proven HSG, a safe, highly effective, and affordable treatment alternative administered by a local podiatrist or dermatologist.

